Search Market Research Report
CAR-T Cell Therapy Market Size, Share Global Analysis Report, 2025 – 2034
CAR-T Cell Therapy Market By End-Users (Cancer Treatment Centers And Hospitals), By Diseases (Multiple Myeloma, Lymphoma, Leukemia, And Others), By Products (Yescarta, Tecartus, Kymriah, Carvykti, Breyanzi, Abecma, And Others), And By Region - Global Industry Insights, Overview, Comprehensive Analysis, Trends, Statistical Research, Market Intelligence, Historical Data and Forecast 2025 – 2034
Industry Insights
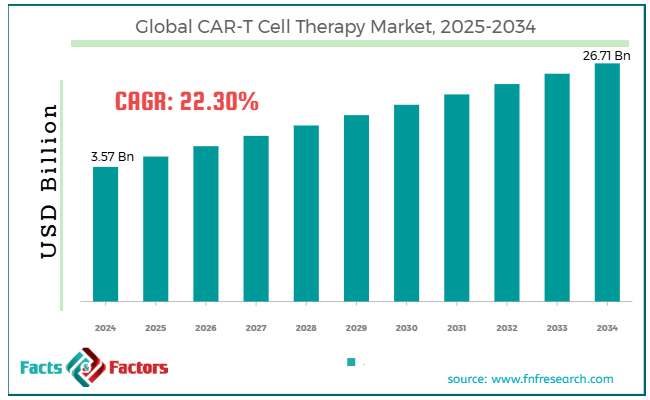
[221+ Pages Report] According to Facts & Factors, the global CAR-T cell therapy market size was valued at USD 3.57 billion in 2024 and is predicted to surpass USD 26.71 billion by the end of 2034. The CAR-T cell therapy industry is expected to grow by a CAGR of 22.30% between 2025 and 2034.
 Market Overview
Market Overview
CAR-T cell therapy stands for Chimeric Antigen Receptor T-cell therapy, a kind of immunotherapy used to treat blood cancer. CAR-T cell therapy involves extracting T cells from the human body, which are further genetically modified. These modified CAR-T cells are multiplied to be infused into the patient’s bloodstream. These infused cells help destroy cancer cells. CAR-T cell therapy is more popular in the market because it is a highly targeted treatment with durable remission rates.
 Key Insights
Key Insights
- As per the analysis shared by our research analyst, the global CAR-T cell therapy market size is estimated to grow annually at a CAGR of around 22.30% over the forecast period (2025-2034).
- In terms of revenue, the global CAR-T cell therapy market size was valued at around USD 3.57 billion in 2024 and is projected to reach USD 26.71 billion by 2034.
- Increasing incidences of hematology cancer are driving the growth of the global CAR-T cell therapy market.
- Based on the end-users, the hospital segment is growing at a high rate and is projected to dominate the global market.
- Based on diseases, the lymphoma segment is anticipated to grow with the highest CAGR in the global market.
- Based on the products, the Yescarta segment is projected to swipe the largest market share.
- Based on region, North America is expected to dominate the global market during the forecast period
 Growth Drivers
Growth Drivers
- Increasing incidences of hematological cancer are driving the growth of the global market.
The high prevalence of lymphoma, multiple myeloma, leukemia, etc., is driving the huge demand for CAR-T cell therapies globally. These therapies serve even those who have failed conventional treatments, which further popularize CAR-T cell therapy.
Additionally, the ever-changing and supporting regulatory landscape is further expected to accelerate the approval of different therapies from organizations like the FDA, NMPA, EMA, and many others. The increasing number of clinical trials is likely to revolutionize the industry further.
Companies are exploring new targets, including multitarget CARs, tumors, and many others, which are further expected to foster numerous growth opportunities in the market.
Also, the rising trend of partnerships between biotech firms and pharma companies to come up with better products and research papers is expected to explore the industry further. Academic institutions and CMOs are also involved in these processes, which is further expected to boost the growth rate of the market.
However, merger and association activities are likely to help with the easy commercialization of these products, which is also a fueling factor in the industry.
The market is likely to see a significant surge in sales figures in the coming years because of the ever-expanding biotech ecosystem globally. West pharma firms are focusing on escalating the development and commercialization process, which is likely to help the market grow further.
The improved access to healthcare facilities, particularly in developing and underdeveloped regions, is another major factor positively influencing the market's growth. Increasing patient access to oncology care infrastructure is also expected to widen the scope of the global CAR-T cell therapy market.
For instance, Autolus Therapeutics plc successfully partnered with Cardinal Health Inc. to commercialize CAR T-cell therapies in the US.
 Restraints
Restraints
- High treatment costs are likely to hinder the growth of the global market.
CAR-T cell therapy is more expensive than other therapies, which is a major factor responsible for the slow growth of the CAR-T cell therapy industry. This therapy has a very limited adoption rate in low and middle-income industries because of the cost burden.
Additionally, the potential side effects of these therapies, like prolonged infection or neurotoxicity, further limit the adoption of these therapies in limited-sourced hospitals.
 Opportunities
Opportunities
- Growing awareness is expected to foster growth opportunities in the global market.
Rising awareness among people regarding early detection and treatment is one of the crucial factors fostering growth opportunities in the global CAR-T cell therapy market. People are increasingly being educated regarding the available cancer treatment, which is further likely to commercialize the products and services involved.
Also, the increasing number of hematologists and oncologists in every city is further resulting in a wider adoption rate. In addition, the growing patient advocacy for CAR-T cell therapy is further strengthening the demand in the market.
The availability of easy reimbursement and insurance coverage policies, particularly in key markets like Japan, Europe, and the US, is further improving the accessibility of patients to these advanced therapies.
Therefore, all these factors are expected to contribute to the industry's growth in the coming years. For instance, Gilead got approval from the FDA for YESCARTA in 2023 to lower the median turnaround time from 16 days to 14 days.
 Challenges
Challenges
- Complex manufacturing processes are a big challenge in the global market.
CAR-T cell therapy usually takes 2 to 4 weeks, starting from the collection of cells to the infusion of modified cells into the human body. Also, sometimes, a delay in the process proves to be very fatal for patients with progressing cancers. Therefore, such a landscape is a big challenge in the CAR-T cell therapy industry.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2024 |
USD 3.57 Billion |
Projected Market Size in 2034 |
USD 26.71 Billion |
CAGR Growth Rate |
22.30% CAGR |
Base Year |
2024 |
Forecast Years |
2025-2034 |
Key Market Players |
GSK plc., Sorrento Therapeutics Inc., Sangamo Therapeutics, Merck & Co. Inc., Bluebird Bio Inc., JW Therapeutics (Shanghai) Co. Ltd., Johnson & Johnson Services Inc., Gilead Sciences Inc., Novartis AG, Bristol-Myers Squibb Company, and others. |
Key Segment |
By End-Users, By Diseases, By Products, and Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East &, Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Segmentation Analysis
Segmentation Analysis
The global CAR-T cell therapy market can be segmented into end-users, diseases, products, and regions.
On the basis of end-users, the market can be segmented into cancer treatment centers and hospitals. The hospital segment is likely to account for the largest share of the global market during the forecast period. Hospitals are an advanced healthcare infrastructure that provides treatment for all diseases, ranging from simple to complex treatments like CAR-T cell therapy.
Also, these infrastructures have specialized personnel like hematologists, oncologists, etc., to serve dedicated patients.
Additionally, these centers have advanced inpatient monitoring capabilities that can help patients monitor the advantages and side effects of the therapy. Hospitals initiate numerous clinical trials for both academic and medical purposes, which further intensifies their dominance in the market. Hospitals have better reimbursement policies, particularly for CAR-T therapies, as these centers work with many government and private insurance companies.
On the basis of disease, the CAR-T cell therapy industry can be segmented into multiple myeloma, lymphoma, leukemia, and others. The lymphoma segment is expected to dominate the global CAR-T cell therapy market during the anticipated period. The increasing incidences of lymphoma all across the globe are a leading reason for the high growth rate of the segment. Nowadays, the fast approvals of advanced CAR-T therapies like Breyanzi and Yescarta are further uplifting the growth of the segment.
Also, the CAR-T cell treatments for lymphoma have better clinical outcomes, such as durable responses and high remission rates, making them an ideal option for patients. The government and medical sector are striving hard to increase the clinical trials for lymphoma, which is further expected to drive the development of the market. Also, the growing awareness among people regarding lymphoma is a leading factor in fostering growth opportunities in the segment.
On the basis of products, the market can be segmented into Yescarta, Tecartus, Kymriah, Carvykti, Breyanzi, Abecma, and others. Yescarta is expected to be the fastest-growing segment in the global market during the forecast period. Yescarta was innovated by Kite Pharma, which is approved for a wide range of lymphoma indications. The product is likely to have better efficacy and durability trials, which will help it position itself better in the marketplace.
Yescarta was the first product for the second line of treatment of LBCL, which is another crucial reason for the dominance of the segment in the CAR-T cell therapy industry. Hospitals and healthcare centers are adopting these products in their treatment plans, which is further expected to support the growth of the segment. In addition, the company's strong distribution network supports the faster commercialization of these products.
 Regional Analysis
Regional Analysis
- North America is to dominate the global market.
North America is likely to account for the largest share of the global CAR-T cell therapy market during the forecast period. The US is a leading market in the region because of its dominance in the CAR-T space. Also, companies are getting approvals for new therapies, which is likely to establish their position in the regional market.
The rapidly expanding biotech infrastructure in the United States is also expected to foster growth opportunities in the CAR-T cell therapy industry. Canada is also witnessing a significant surge in market revenue because of the increasing healthcare spending in the region. Modifications in the regulatory landscape are also expected to support the rapid approval of therapies. However, North America is witnessing rising incidences of cancer, which is driving huge revenues in the market.
Also, the strong presence of market players is further expected to support the growth trajectory of the regional market. In addition, North America has the most favorable investment policies, which are anticipated to encourage people to adopt CAR-T therapies on a larger scale. Government organizations and big pharma companies are investing heavily in initiating clinical trials to develop more advanced cell therapy, which, in turn, is expected to revolutionize the market in the coming years.
Asia Pacific is another growing region that is expected to witness a strong growth trajectory in the coming years. China is boosting the region's sales revenue with major investments in CAR-T cell therapy. The country is also witnessing the approval of therapies like CAR-T cell therapy, which is further expected to revolutionize the regional market. Japan is also strengthening its position in the regional market with the invention of precision medicine and the innovation of CAR-T cell therapies by companies like Takeda.
Furthermore, India is likely to emerge as a leading region in the coming years because of the growing government-backed research activities. Also, there are many therapies in the CAR-T cell space in India that are in the early stage, but are likely to increase domestic revenue. For instance, Abecma got approval from the FDA in 2021 for people with multiple myeloma for the purpose of treating cancer patients.
 Competitive Analysis
Competitive Analysis
The key players in the global CAR-T cell therapy market include:
- GSK plc.
- Sorrento Therapeutics Inc.
- Sangamo Therapeutics
- Merck & Co. Inc.
- Bluebird Bio Inc.
- JW Therapeutics (Shanghai) Co. Ltd.
- Johnson & Johnson Services Inc.
- Gilead Sciences Inc.
- Novartis AG
- Bristol-Myers Squibb Company
For instance, Wisconsin Alumni Research Foundation (WARF) successfully collaborated with Ginkgo Bioworks to come up with GD2 CAR T-cell Therapies.
The global CAR-T cell therapy market is segmented as follows:
 By End-Users Segment Analysis
By End-Users Segment Analysis
- Cancer Treatment Centers
- Hospitals
 By Diseases Segment Analysis
By Diseases Segment Analysis
- Multiple Myeloma
- Lymphoma
- Leukemia
- Others
 By Products Segment Analysis
By Products Segment Analysis
- Yescarta
- Tecartus
- Kymriah
- Carvykti
- Breyanzi
- Abecma
- Others
 By Regional Segment Analysis
By Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- Southeast Asia
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Industry Major Market Players
- GSK plc.
- Sorrento Therapeutics Inc.
- Sangamo Therapeutics
- Merck & Co. Inc.
- Bluebird Bio Inc.
- JW Therapeutics (Shanghai) Co. Ltd.
- Johnson & Johnson Services Inc.
- Gilead Sciences Inc.
- Novartis AG
- Bristol-Myers Squibb Company

Copyright © 2025 - 2026, All Rights Reserved, Facts and Factors


