Search Market Research Report
Viral Vector and Plasmid DNA Manufacturing Market Size, Share Global Analysis Report, 2018 – 2027
Viral Vector and Plasmid DNA Manufacturing Market By Product (Viral Vectors and Plasmid), By End-User (Biopharmaceutical Companies and Research Institutes), and By Application (Gene & Cancer Therapies, Formulation Development, Viral Infections, and Immunotherapy): Global Industry Perspective, Comprehensive Analysis, and Forecast 2018 – 2027
Industry Insights
The report covers the forecast and analysis of the viral vector and plasmid DNA manufacturing market on a global and regional level. The study provides historical data from 2015 to 2018 along with a forecast from 2019 to 2027 based on revenue (USD Million). The study includes drivers and restraints of the viral vector and plasmid DNA manufacturing market along with the impact they have on the demand over the forecast period. Additionally, the report includes the study of opportunities available in the viral vector and plasmid DNA manufacturing market on a global level.
In order to give the users of this report a comprehensive view of the viral vector and plasmid DNA manufacturing market, we have included a competitive landscape and an analysis of Porter’s Five Forces model for the market. The study encompasses a market attractiveness analysis, wherein all the segments are benchmarked based on their market size, growth rate, and general attractiveness.
The report provides company market share analysis to give a broader overview of the key players in the market. In addition, the report also covers key strategic developments of the market including acquisitions & mergers, new product & service launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the market on a global and regional basis.
 Report Scope
Report Scope
Report Attributes |
Details |
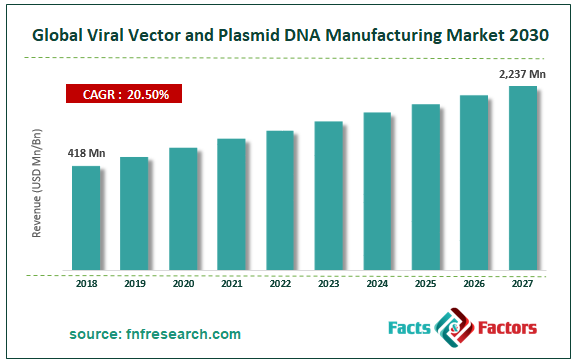
Market Size in 2018 |
USD 418 Million |
Projected Market Size in 2027 |
USD 2,237 Million |
CAGR Growth Rate |
20.5 % CAGR |
Base Year |
2017 |
Forecast Years |
2018 – 2027 |
Key Market Players |
Kaneka Corporation (Eurogentec), Cobra Biologics, VGXI, Inc., DNA manufacturing market include Lonza, FUJIFILM Diosynth Biotechnologies Inc., Genzyme Corporation, Vigene Biosciences Inc., Brammer Bio, Oxford Gene Technology, SIRION Biotech GmbH, FinVector Vision Therapies, VIROVEK, Novasep, SPARK THERAPEUTICS, INC., ALDEVRON, and General Electric Company (GE Healthcare). |
Key Segment |
By Product, By Application, By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
The study provides a decisive view of the viral vector and plasmid DNA manufacturing market by segmenting the market based on the product, end-user, application, and regions. All the segments have been analyzed based on present and future trends and the market is estimated from 2019 to 2027. The regional segmentation includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
The rise in the allocation of funds by private organizations for carrying out research activities is predicted to steer the market expansion over the forecast timespan. In addition to this, manufacturers are implementing new technologies like cell line culture development, expression systems, and cell culture system for effectively handling activities related to viral-based vector development. All these aspects will upsurge market growth during the forecast period. Nonetheless, the risk of mutagenesis & other obstructions in gene therapy as well as huge costs associated with gene treatment will put brakes on the growth of the market over the forecast period.
Based on the product, the market is divided into viral Vectors and plasmid. In terms of end-user, the market is sectored into Biopharmaceutical Companies and Research Institutes. Application-wise, the industry is classified into Gene & Cancer Therapies, Formulation Development, Viral Infections, and Immunotherapy.
 Some of the key participants in the viral vector and plasmid DNA manufacturing business are
Some of the key participants in the viral vector and plasmid DNA manufacturing business are
- Kaneka Corporation (Eurogentec)
- Cobra Biologics
- VGXI Inc.
- DNA manufacturing market include Lonza
- FUJIFILM Diosynth Biotechnologies Inc.
- Genzyme Corporation
- Vigene Biosciences Inc.
- Brammer Bio
- Oxford Gene Technology
- SIRION Biotech GmbH
- FinVector Vision Therapies
- VIROVEK
- Novasep
- SPARK THERAPEUTICS INC.
- ALDEVRON
- General Electric Company (GE Healthcare)
Industry Major Market Players
- Kaneka Corporation (Eurogentec)
- Cobra Biologics
- VGXI Inc.
- Lonza
- FUJIFILM Diosynth Biotechnologies Inc.
- Genzyme Corporation
- Vigene Biosciences Inc.
- Brammer Bio
- Oxford Gene Technology
- SIRION Biotech GmbH
- FinVector Vision Therapies
- VIROVEK, Novasep
- SPARK THERAPEUTICS Inc.
- ALDEVRON
- General Electric Company (GE Healthcare)
Frequently Asked Questions

Copyright © 2025 - 2026, All Rights Reserved, Facts and Factors


