Search Market Research Report
Pharmacovigilance Market Size, Share Global Analysis Report, , 2020 – 2026
Pharmacovigilance Market By Service Provider (In-house, and Contract Outsourcing), By Product Life Cycle (Pre-clinical, Phase I, Phase II, Phase III, and Phase IV), By Type (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, and EHR Mining), By Process Flow (Case Data Management, Signal Detection, and Risk Management System), By Therapeutic Area (Oncology, Neurology, Cardiology, Respiratory Systems, and Others), and By End user (Pharmaceuticals, Biotechnology Companies, Medical Device Manufacturers, and Others)Global Industry Perspective, Comprehensive Analysis, and Forecast, 2020 – 2026
Industry Insights
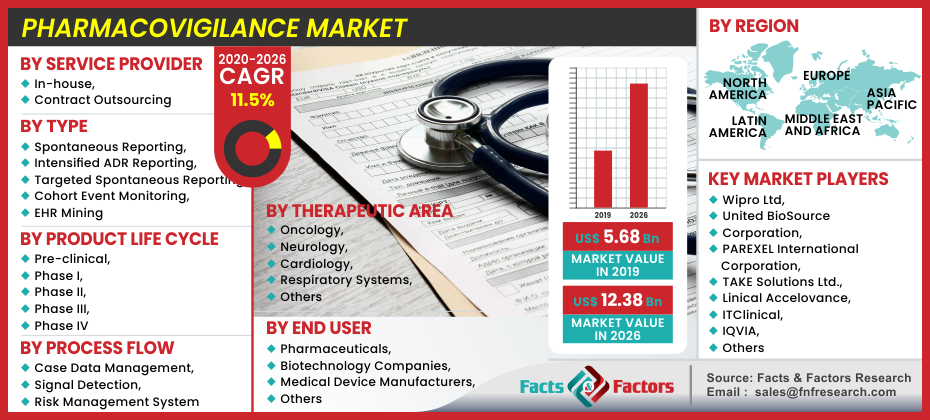
[225+ Pages Report] Global Pharmacovigilance market was projected at USD 5.68 Billion in 2019, which is expected to hit USD 12.38 Billion in 2026 and is expected to rise to CAGR by 11.5% between 2020 and 2026.
 Market Overview
Market Overview
Patient treatment and wellbeing with regards to the use of drugs are the main focus of the Pharmacovigilance PV sector. It also promotes public health programs, through the provision of accurate and balanced information for the proper evaluation of drugs' harmful consequences. The PV industry includes a number of tasks such as the identification, monitoring, and analysis of adverse drug reactions in the production of the requisite data points for regulatory activities. In addition, increased patient population use of medications combined with increased drug safety standards is the key contributor to growth in the photovoltaics industry in the coming few years. In terms of sales and growth, PV outsourcing gains traction because of progress in PV technologies and services. A world economy has been paved with the existence of a forum for information technology. Moreover, in order to invent new vaccines that have launched many clinical trials, the advent of COVID-19 has opened up several possibilities for pharmaceutical firms The photovoltaics market is also opened because it is the main step in any process of drugs production.
 Industry Growth Factors
Industry Growth Factors
Security and feasibility are the two key criteria that must be passed during any step of the clinical trial. Populations today are more mindful of the side effects of OTC drugs and thus require safer prescriptions. The monitoring of branded drugs for potential ADRs would certainly improve this market benefit. In addition, government policies, and awareness programs for pharmaceutical firms have a larger influence on the dissemination of accurate medicinal knowledge, the safe use of medicinal drugs, the side effects of medicinal products, etc. The Uppsala Monitoring Center (UMC), for example, operates a social media program every year to enable patients and the general population to be aware of the proper use of drugs and update on suspicious ADRs. In 2019, UMC initiated a program aimed at pharmaceutical production and the healthy use of drugs for seniors. These good policies are also likely during the projected period to encourage development and growth in this sector. The current competition in Pharmacovigilance offers an option to outsource the PV operation for pharmaceutical products and hospitals in the context of time limitations and services that an outsourced operation can provide; PV outsourcing has optimum benefits for production firms. Outsourcing also enables businesses to be customized to their needs, thereby increasing the tempo of the assessment process. Due to the benefits of outsourcing services, the market for these services is growing and numerous outsourcing opportunities for providers have been created. These providers concentrate on expanding their business portfolio in order to provide consumers with more advanced solutions. Growing demand for outsourcing at a minimum cost thus leads to the advent of new players on this market, which accelerates market growth from 2020 to late 2026.
 Global Pharmacovigilance Market: Segmentation
Global Pharmacovigilance Market: Segmentation
Based on service provider market is sub-segmented into contract outsourcing, and in-house. Based on the product life cycle market is divided into phase II, Phase I, Phase III, Phase IV, and Pre-clinical. Based on type market is divided into targeted spontaneous reporting, intensified ADR reporting, EHR mining, spontaneous reporting, and cohort event monitoring. Based on process flow the market is divided into signal detection, risk management system, and case data management. Based on therapeutic area market is divided into oncology, respiratory systems, cardiology, neurology, and others. Based on end-users the market is further divided into pharmaceutical, medical device manufacturers, biotechnology companies, and others.
 Global Pharmacovigilance Market: Regional Analysis
Global Pharmacovigilance Market: Regional Analysis
On the regional front, the pharmacovigilance market is classified into North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa. Because of the largest number of PV operations in the country, North America leads this market share. The vast number of deaths caused by ADRs leading to the stringent need for PV was combined with this. In addition, the area has well-established PV centers for the management of its operations with advanced tools. The emphasis on the risk management of pharmaceutical drugs makes Europe the second-largest consumer place. Various tracking programs have been developed in the country, including the European Medicines Agency, EudraVigilance, and others to access and control ADRs.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2019 |
USD 5.68 Billion |
Projected Market Size in 2026 |
USD 12.38 Billion |
CAGR Growth Rate |
11.5% |
Base Year |
2019 |
Forecast Years |
2020-2026 |
Key Market Players |
Wipro Ltd, United BioSource Corporation, PAREXEL International Corporation, TAKE Solutions Ltd., Linical Accelovance, ITClinical, IQVIA, ICON PLC, IBM Corporation, Foresight Group International AG, FMD K&L Inc., Cognizant, Capgemini, BioClinica, ArisGlobal, Accenture, and Others. |
Key Segment |
By Service Provider, By Product Life, By Process Flow, By Therapeutic Area, By End user |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Global Pharmacovigilance Market: Competitive Players
Global Pharmacovigilance Market: Competitive Players
Some of the key players in the Pharmacovigilance Market are
- Wipro Ltd
- United BioSource Corporation
- PAREXEL International Corporation
- TAKE Solutions Ltd.
- Linical Accelovance
- ITClinical
- IQVIA
- ICON PLC
- IBM Corporation
- Foresight Group International AG
- FMD K&L Inc.
- Cognizant
- Capgemini
- BioClinica
- ArisGlobal
- Accenture
 Global Pharmacovigilance Market: Regional Segment Analysis
Global Pharmacovigilance Market: Regional Segment Analysis
- North America
- U.S.
- Canada
- Europe
- U.K.
- France
- Germany
- The Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Mexico
- The Middle East and Africa
Industry Major Market Players
- Wipro Ltd
- United BioSource Corporation
- PAREXEL International Corporation
- TAKE Solutions Ltd.
- Linical Accelovance
- ITClinical
- IQVIA
- ICON PLC
- IBM Corporation
- Foresight Group International AG
- FMD K&L Inc.
- Cognizant
- Capgemini
- BioClinica
- ArisGlobal
- Accenture
Frequently Asked Questions

Copyright © 2023 - 2024, All Rights Reserved, Facts and Factors


