Search Market Research Report
E-Clinical Solutions Market Size, Share Global Analysis Report, , 2021 – 2026
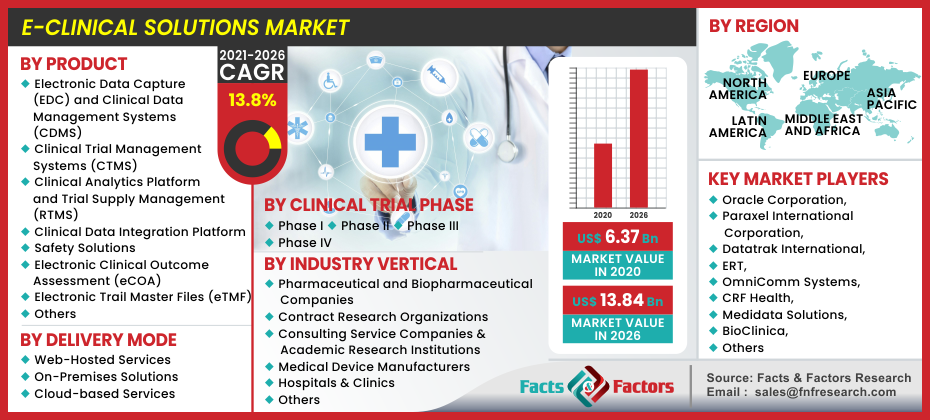
E-Clinical Solutions Market By Product (Electronic Data capture (EDC) and Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Clinical Analytics Platform and Trial Supply Management (RTMS), Clinical Data Integration Platform, Safety Solutions, Electronic Clinical Outcome Assessment (eCOA), Electronic Trail Master Files (eTMF), and Others), By Delivery Mode (Web-Hosted Services, On-Premises Solutions, & Cloud-based Services), By Clinical Phase Trials (Phase I, Phase II, Phase III, & Phase IV), By Industry Verticals (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies & Academic Research Institutions, Medical Device Manufacturers, and Hospitals & clinics, and Others), and By Regions - Global Industry Perspective, Comprehensive Analysis, and Forecast, 2021 – 2026
Industry Insights
[ 213+ Pages Report] As per the latest research and survey report issued by Facts and Factors, the global E-Clinical Solutions market was valued at around USD 6.37 Billion in 2020 and is expected to register revenues worth USD 13.84 Billion by the end of 2026, growing at an exceptional CAGR of approximately 13.8% between 2021 and 2026.
 Market Overview
Market Overview
E-Clinical Solutions are packages that bind together the technical expertise for the smooth functioning of any clinical trial operation. Generally speaking, the E-Clinical solutions are deemed as the software applications that are used in a litany of clinical studies for the purposes of accelerated development. They help with maintaining, recording, and tracking the due dates for any given procedure. E-Clinical Solutions aims to provide clear and precise emulsions w.r.t the course of action they are being utilized upon.
 Industry Growth Factors
Industry Growth Factors
A trend of increasing spending on R&D is observed by pharma-biotech companies coupled with IT-related solutions for the betterment of their products and services. Additionally, increase in procedural costs along with stringent regulatory policies associated with clinical trial studies or research, the demand for E-clinical solutions has been on a steady rise. Furthermore, excess government grants are being provided in order to comply with the dispatched ordinances.
Development of various software applications is gaining acceptance within clinical trials of international modulations which further pushes the demand for e-clinical solutions. Other reasons for growth in revenue can be accredited toward higher quality and concise data, adherence to imposed guidelines by government bodies, and approved and certified solutions by manufacturers and vendors. With the growing risks of heart diseases, cancer, diabetes, and other fatal diseases on the rise, extensive and well-funded clinical trials are well within the scope of the solutions and services e-clinical solutions aim to provide. However, the inadequacy of professionals and patients along with security risks involving confidential data can restrain the market growth.
 Segmentation Analysis
Segmentation Analysis
The global e-clinical solutions market is segregated based on product, clinical phase, delivery mode, industry verticals, and regions.
The product segment can be further broken down into electronic data capture (EDC) and clinical data management systems (CDMS), clinical trial management systems (CTMS), clinical analytics platform and trial supply management (RTMS), clinical data integration platform, safety solutions, electronic clinical outcome assessment (eCOA), electronic trail master files (eTMF), and others. The electronic data capture (EDC) subcategory is expected to capture the largest market share in the category owing to its commonly used applications in clinical trials. Furthermore, the EDC systems are designed keeping in mind for storing trial data in a secure electronic format which increases its usability in a variety of CRO and biopharmaceutical firms.
The delivery mode segment can be further disintegrated into web-hosted services, on-premises solutions & cloud-based services. The web-hosted services will occupy the largest market share due to their additional benefits of ease of access, functionality, and lower cost over their counterparts. However, cloud-based services are expected to witness the highest growth CAGR during the forecast period. Based on the clinical trial phase the segment can be further split between phase I, phase II, phase III, and phase IV. Phase III of the category will grab the largest market share due to its significant position in any kind of clinical trial. With an increased solution for constant randomization for pure results as well as verifying constructive evidence gathered from the past two stages plays an important role in upscaling the likeability of the latter. On the basis of industry verticals the market is segmented among pharmaceutical and biopharmaceutical companies, contract research organizations, consulting service companies & academic research institutions, medical device manufacturers, and hospitals & clinics. The CRO and pharmaceutical and biopharmaceutical segments will occupy a similar market share in the category. Additionally, the cost advantages of outsourcing clinical trials to CRO’s increase efficiency, productivity and enforces the trial conductors to focus on pivotal areas without being too weary about the minute details.
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2020 |
USD 6.37 Billion |
Projected Market Size in 2026 |
USD 13.84 Billion |
CAGR Growth Rate |
13.8% CAGR |
Base Year |
2020 |
Forecast Years |
2021-2026 |
Key Market Players |
Oracle Corporation, Paraxel International Corporation, Datatrak International, ERT, OmniComm Systems, CRF Health, Medidata Solutions, BioClinica, MaxisIT, and ERT Clinical, amongst others. |
Key Segment |
By Product, By Delivery Mode, By Clinical Trial Phase, By Industry Vertical, and By Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Regional Analysis
Regional Analysis
North America is expected to dominate the regional overview followed by the Asia-Pacific region. The major factor attributed to the growth of the e-clinical solutions market in the region is accredited to the extensive drug developmental cycle. Furthermore, the early adoption of e-clinical solutions and a swift transfer from traditional practices to real-time analytical solutions has increased the foothold of the market.
Deploying clinical trial studies toward large pharmaceutical and biopharmaceutical organizations in the Asia-Pacific region, followed by a rise in government funding for the same has propelled the growth for the latter. Furthermore, the region boasts of less stringent regulation compared to other nations in terms of setting up clinical trials, acceptance of larger subjects, and the quality of recruitment of subjects for clinical studies.
 Competitive Players
Competitive Players
Some main participants of the global E-Clinical Solutions market are :
- Oracle Corporation
- Paraxel International Corporation
- Datatrak International
- ERT
- OmniComm Systems
- CRF Health
- Medidata Solutions
- BioClinica
- MaxisIT
- ERT Clinical
 By Product Segment Analysis
By Product Segment Analysis
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platform and Trial Supply Management (RTMS)
- Clinical Data Integration Platform
- Safety Solutions
- Electronic Clinical Outcome Assessment (eCOA)
- Electronic Trail Master Files (eTMF)
- Others
 By Delivery Mode Segment Analysis
By Delivery Mode Segment Analysis
- Web-Hosted Services
- On-Premises Solutions
- Cloud-based Services
 By Clinical Trial Phase Segment Analysis
By Clinical Trial Phase Segment Analysis
- Phase I
- Phase II
- Phase III
- Phase IV
 By Industry Vertical Segment Analysis
By Industry Vertical Segment Analysis
- Pharmaceutical and Biopharmaceutical Companies
- Contract Research Organizations
- Consulting Service Companies & Academic Research Institutions
- Medical Device Manufacturers
- Hospitals & Clinics
- Others
 By Regional Segment Analysis
By Regional Segment Analysis
- North America
- U.S.
- Canada
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- The Middle East and Africa
- GCC Countries
- South Africa
- Rest of MEA
Industry Major Market Players
- Oracle Corporation
- Paraxel International Corporation
- Datatrak International
- ERT
- OmniComm Systems
- CRF Health
- Medidata Solutions
- BioClinica
- MaxisIT
- ERT Clinical
Frequently Asked Questions

Copyright © 2023 - 2024, All Rights Reserved, Facts and Factors


