Search Market Research Report
Blood Clot Retrieval Devices Market Size, Share Global Analysis Report, 2023 – 2030
Blood Clot Retrieval Devices Market Size, Share, Growth Analysis Report By Stroke (Ischemic Stroke (Blood Clot), Hemorrhagic Stroke (Rupturing of Arteries) and Transient Ischemic Attack), By Device (Mechanical Embolus Removal Devices, Penumbra Blood Clot Retrieval Devices, Stent Retrievers, Aspiration Devices, and Ultrasound Assisted Devices), Application (Coronary Arteries, Peripheral Arteries, and Cerebral Arteries), By End User (Hospitals, Diagnostic Centers, Clinics and Ambulatory Surgical Centers), and By Region - Global and Regional Industry Insights, Overview, Comprehensive Analysis, Trends, Statistical Research, Market Intelligence, Historical Data and Forecast 2023 – 2030
Industry Insights
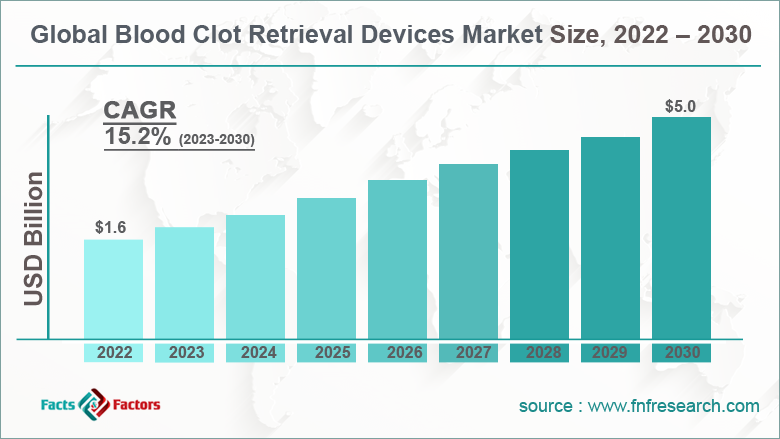
[227+ Pages Report] According to the report published by Facts and Factors, the global blood clot retrieval devices market size was worth around USD 1.6 billion in 2022 and is predicted to grow to around USD 5.0 billion by 2030 with a compound annual growth rate (CAGR) of roughly 15.2% between 2023 and 2030. The report analyzes the global blood clot retrieval devices market drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the blood clot retrieval devices industry.
 Market Overview
Market Overview
The more technical term for devices that retrieve blood clots is thrombectomy devices. Thrombectomy is an interventional procedure that is typically performed on the coronary arteries, peripheral arteries, and brain arteries. A stroke is a medical condition that causes the loss of brain cells as a direct result of insufficient blood supply. A blood clot retrieval procedure involves mechanically removing a blood clot from the patient's brain. This procedure is used on patients who have had a stroke. This procedure is most commonly used to treat ischemic strokes, which are caused by blood clots.
 Key Insights
Key Insights
- As per the analysis shared by our research analyst, the global blood clot retrieval devices market is estimated to grow annually at a CAGR of around 15.2% over the forecast period (2023-2030).
- In terms of revenue, the global blood clot retrieval devices market size was valued at around USD 1.6 billion in 2022 and is projected to reach USD 5.0 billion, by 2030.
- The increasing prevalence of stroke across the globe is expected to drive the growth of the blood clot retrieval devices industry over the projected period.
- Based on the stroke, the ischemic stroke (blood clot) segment is expected to dominate the market over the forecast period.
- Based on the device, the mechanical embolus removal devices segment is expected to dominate the market over the forecast period.
- Based on region, North America is expected to dominate the market during the forecast period.
 Growth Drivers
Growth Drivers
- The growing prevalence of stroke across the globe drives the market growth
The growing prevalence of stroke across the globe is expected to drive the growth of the global blood clot retrieval devices market during the forecast period. Stroke is one of the leading causes of death worldwide. According to the CDC, a stroke will account for one in every six CVD deaths in 2020, and one person in the United States will have a stroke every 40 seconds.
As a result, the demand for effective and efficient treatment solutions is expected to rise in tandem with the number of stroke victims. Blood clot retrieval devices treat stroke patients effectively and improve patient care. As a result, the demand for blood clot retrieval devices is constantly increasing.
 Restraints
Restraints
- The lack of skilled professionals acts as a major restraint
With the aid of surgical procedures, the use of blood clot retrieval tools also entails the use of extremely cutting-edge technologies. With the aid of these cutting-edge tools, surgical operations can only be carried out by highly qualified and experienced personnel. During the forecast period, the blood clot retrieval devices industry's development will be constrained by a lack of skilled and trained workers to operate these sophisticated devices.
 Opportunities
Opportunities
- Favorable reimbursement policies provide a lucrative opportunity
In developed nations, many insurance companies have incorporated the price of these treatments into their policies. For instance, in the United States in 2018, the Centers for Medicare and Medicaid Services (CMS) increased funding for these procedures. These devices and procedures are now available to many people due to these advantageous reimbursement policies. The benefits of these devices are becoming more and more known to patients, which is anticipated to support blood clot retrieval devices market expansion. Additionally, people's preference for thrombectomy procedures has increased recently, which is anticipated to fuel market growth.
 Challenges
Challenges
- The high cost of devices poses a major challenge
The high expense of these cutting-edge technologies for unclogging clogged vascular systems and removing blood clots presents a challenge to the market's expansion during the forecast period. These tools and surgeries are out of the reach of less developed and economically backward nations. Thus, acting as a major challenge for industry expansion.
 Segmentation Analysis
Segmentation Analysis
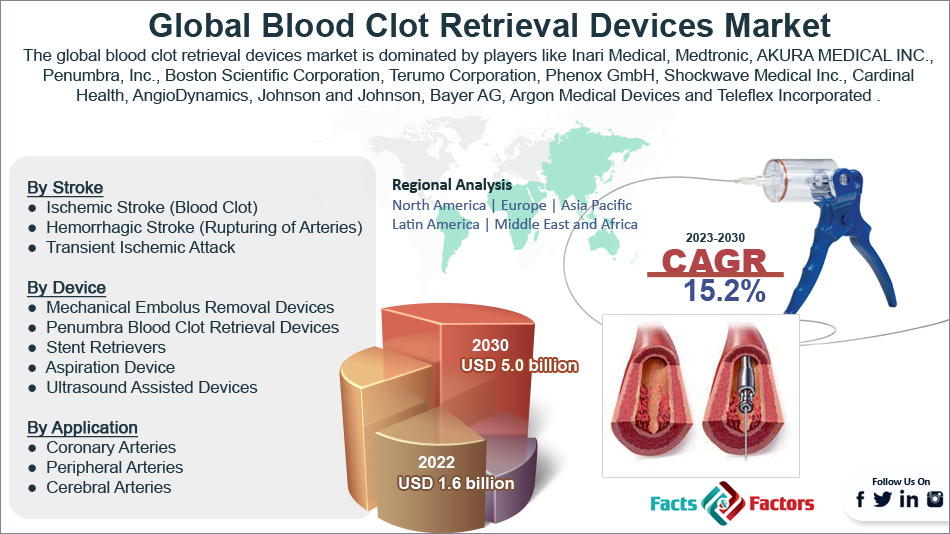
The global blood clot retrieval devices industry is segmented based on stroke, device, application, end user, and region.
Based on the stroke, the global market is bifurcated into ischemic stroke (blood clot), hemorrhagic stroke (rupturing of arteries), and transient ischemic attack. The ischemic stroke (blood clot) segment is expected to dominate the market over the forecast period. The growth in the segment is attributed to the high prevalence of these strokes across the globe. An ischemic stroke occurs when a blood vessel is obstructed, typically by a blood clot, depriving part of the brain of oxygen. Ischemic strokes account for about 87% of all strokes and are the most frequent form. 32,000 brain cells per second pass away, and 1.9 million in 59 seconds after an ischemic stroke. The hemorrhagic stroke (rupturing of arteries) segment is growing at the highest CAGR over the forecast period.
According to NCBI, 10% to 20% of strokes each year are hemorrhagic strokes. In the United States of America, the United Kingdom, and Australia, hemorrhage occurs in strokes at a rate of 8–15%, whereas in Japan and Korea, it occurs at a rate of 18–24%. The frequency ranges from 12% to 15% per 100,000 people per year. Asians and people from low- and middle-income nations are particularly affected. The incidence rises with age and is more prevalent in males. The prevalence is rising worldwide, primarily in Asian and African nations. According to Japanese statistics, ICH is less common when hypertension is under control. In high-income nations, the case mortality rate ranges from 25% to 30%, while in low- to middle-income nations, it ranges from 30% to 48%. The effectiveness of critical treatment affects the ICH fatality rate. Thus, driving segmental growth.
Based on the application, the global market is bifurcated into coronary arteries, peripheral arteries, and cerebral arteries.
Based on the end user, the market is bifurcated into hospitals, diagnostic centers, clinics, and ambulatory surgical centers.
Based on the device, the global blood clot retrieval devices industry is segmented into mechanical embolus removal devices, penumbra blood clot retrieval devices, stent retrievers, aspiration devices, and ultrasound-assisted devices. The mechanical embolus removal devices segment is expected to dominate the market over the forecast period. When compared to all the other blood clot retrieval devices on the market, this one is incredibly affordable.
In the upcoming years, it is anticipated that this device will contribute significantly to the development of the global blood clot retrieval device market. The lifespan of a patient after surgery is anticipated to rise with the use of blood clot retrieval devices. These devices are anticipated to have a bigger market share and to be in high demand during the forecast period due to the increase in survival time caused by their use compared to previously used medical care.
Moreover, due to the rising geriatric population in both developed and developing economies, the use of these devices is anticipated to increase in the upcoming years. The market is anticipated to expand as a result of rising knowledge about the availability of these devices for surgery. All medical institutions will make use of the devices to treat acute ischemic stroke. This will encourage market expansion in developing countries where reimbursement policies are available.
 Recent Developments:
Recent Developments:
- In February 2022, the latest advancement in the company's effort to improve stroke care, Zoom POD Aspiration Tubing, was introduced by Imperative Care, Inc. The Zoom POD is the most recent addition to Imperative Care's Zoom Stroke Solution, a portfolio of products for treating ischemic stroke that also includes the Zoom 88 Large Distal Platform for neurovascular access, four different-sized Zoom Aspiration Catheters, and the Zoom Pump with accessories. The Zoom POD is the first and only sterile field clot filter device, providing patients with ischemic strokes with a quicker time to clot capture during crucial mechanical thrombectomy operations.
- In March 2022, a fully owned subsidiary of a major provider of medical technology, India Medtronic Private Limited, announced the opening of the nation's first-ever dedicated registry for the gathering of information about the use of revascularization devices in patients with acute ischemic stroke (AIS). An industry-first initiative, the Prospective Registry for Assessment of Acute Ischemic Stroke Patients Treated with Neurothrombectomy Devices in India (PRAAN), aims to establish a post-market registry to evaluate clinical outcomes related to the use of Medtronic market-released revascularization devices in patients suffering from acute ischemic stroke.
- In February 2022, Innova Vascular, Inc. and Cardiovascular Systems, Inc. (CSI) collaborated on developing a complete range of thrombectomy devices, according to CSI. The company claims that CSI plans to purchase and sell novel thrombectomy devices from Innova that are intended to treat peripheral vascular diseases, such as deep vein thrombosis (DVT) and pulmonary embolism (PE).
 Report Scope
Report Scope
Report Attribute |
Details |
Market Size in 2022 |
USD 1.6 Billion |
Projected Market Size in 2030 |
USD 5 Billion |
CAGR Growth Rate |
15.2% CAGR |
Base Year |
2022 |
Forecast Years |
2023-2030 |
Key Market Players |
Inari Medical, Medtronic, AKURA MEDICAL INC., Penumbra Inc., Boston Scientific Corporation, ZYLOX-TONBRIDGE MEDICAL TECHNOLOGY CO. LTD., Terumo Corporation, Phenox GmbH, Shockwave Medical Inc., Cardinal Health, AngioDynamics, Johnson and Johnson, Bayer AG, Argon Medical Devices, Teleflex Incorporated, and others. |
Key Segment |
By Stroke, Device, Application, End User, and Region |
Major Regions Covered |
North America, Europe, Asia Pacific, Latin America, and the Middle East &, Africa |
Purchase Options |
Request customized purchase options to meet your research needs. Explore purchase options |
 Regional Analysis
Regional Analysis
- North America is expected to dominate the market during the forecast period
North America is expected to dominate the global blood clot retrieval devices market during the forecast period. The growth in the region is attributed to the high prevalence of stroke. A stroke is a serious medical condition that necessitates immediate medical attention and can result in permanent brain damage, long-term disability, or even death. Stroke is the fifth leading cause of death in the United States, and it is a major cause of serious disability in adults, according to the Centers for Disease Control and Prevention. Every year, approximately 795,000 people in the United States suffer a stroke. Ischemic strokes represent about 87 percent of all strokes. Moreover, the growing FDA approvals regarding the use of these devices are expected to flourish the market growth.
For instance, in February 2018, The FDA approved the use of the Trevo clot retrieval device to treat certain stroke patients up to 24 hours after symptom onset, broadening the device's indication to a larger group of patients. This device has been cleared for use as an initial therapy for strokes caused by blood clots (also known as an acute ischemic stroke) to reduce paralysis, speech difficulties, and other stroke disabilities, but only in conjunction with treatment with tissue plasminogen activator, a medication that dissolves blood clots (t-PA). The device had previously been cleared for use in patients six hours after the onset of symptoms. Thus, the growing prevalence of stroke and the approval of these devices is expected to fuel the market expansion in the region.
The Asia Pacific is expected to grow at the highest CAGR over the forecast period. The market will grow as a result of increased government expenditure on healthcare infrastructure in the Asia Pacific. To better service their citizens, India, China, and Australia all make significant infrastructure investments. The expenditure made in the Union Budget demonstrates how far India has come. India's healthcare industry is developing quickly. The medical industry expands as a result of altered government regulations, increased investment, and mergers.
For instance, according to the India Brand Equity Foundation, the Indian healthcare industry is anticipated to expand by three times, at a CAGR of 22% between 2016 and 2022, from US$ 110 billion to US$ 372 billion. Indian healthcare infrastructure is anticipated to reach $349.1 billion by FY22. Moreover, India's public healthcare spending was 2.1% of GDP in 2021–2022, up from 1.8% in 2020–2021 and 1.3% in 2019–20, according to the Economic Survey of 2022. Thus, the aforementioned factor is expected to flourish the market in the region.
 Competitive Analysis
Competitive Analysis
- Inari Medical
- Medtronic
- AKURA MEDICAL INC.
- Penumbra Inc.
- Boston Scientific Corporation
- ZYLOX-TONBRIDGE MEDICAL TECHNOLOGY CO. LTD.
- Terumo Corporation
- Phenox GmbH
- Shockwave Medical Inc.
- Cardinal Health
- AngioDynamics
- Johnson and Johnson
- Bayer AG
- Argon Medical Devices
- Teleflex Incorporated
The global blood clot retrieval devices market is segmented as follows:
 By Stroke Segment Analysis
By Stroke Segment Analysis
- Ischemic Stroke (Blood Clot)
- Hemorrhagic Stroke (Rupturing of Arteries)
- Transient Ischemic Attack
 By Device Segment Analysis
By Device Segment Analysis
- Mechanical Embolus Removal Devices
- Penumbra Blood Clot Retrieval Devices
- Stent Retrievers
- Aspiration Device
- Ultrasound Assisted Devices
 By Application Segment Analysis
By Application Segment Analysis
- Coronary Arteries
- Peripheral Arteries
- Cerebral Arteries
 By End User Segment Analysis
By End User Segment Analysis
- Hospitals
- Diagnostic Centers
- Clinics
- Ambulatory Surgical Centers
 By Regional Segment Analysis
By Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Nordic Countries
- Denmark
- Sweden
- Norway
- Benelux Union
- Belgium
- The Netherlands
- Luxembourg
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Southeast Asia
- Indonesia
- Thailand
- Malaysia
- Singapore
- Rest of Southeast Asia
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Industry Major Market Players
- Inari Medical
- Medtronic
- AKURA MEDICAL INC.
- Penumbra Inc.
- Boston Scientific Corporation
- ZYLOX-TONBRIDGE MEDICAL TECHNOLOGY CO. LTD.
- Terumo Corporation
- Phenox GmbH
- Shockwave Medical Inc.
- Cardinal Health
- AngioDynamics
- Johnson and Johnson
- Bayer AG
- Argon Medical Devices
- Teleflex Incorporated
Frequently Asked Questions

Copyright © 2023 - 2024, All Rights Reserved, Facts and Factors


